Stephanie Lenck is the first physician researcher to have understood the role of the venolymphatic system in IIH. In this article written for IIH-Hub, she summarizes her findings, first published in 2018 in the Journal Neurology (reference 6).
The recent discoveries of the glymphatic system and of dural lymphatic vessels represent major breakthroughs in basic neurosciences, allowing new insights into complex neurological conditions1-5. In light of these findings, the interplay between the circulation of CSFFluid that is made by specialized cells in the ventricles of the brain. Click the term to read more and the cerebrovascular system (including arteries, veins and lymphatics) seems to be more intimately related than previously suspected. In the broader context of these basic science findings, idiopathicThe term idiopathic is used when there is no detectable reason for something. Click the term to read more intracranial hypertension (IIH) has been recently summarized in the following pathological triad: “restriction of the venous CSF outflow pathway – overflow of the lymphatic CSF outflow pathway – congestion of the glymphatic system”6, 7. Lateral sinus stenoses (LSS) may thus represent the macroscopic evidence of the microscopic restriction in the venous CSF outflow pathway in IIH. These stenoses lead to an increase in the cerebral venous pressure, thereby interrupting the passive resorption of CSF from the subarachnoid space to the venous blood of the dural sinuses. These stenoses thus seem to play a crucial role in maintaining and/or triggering the ‘vicious cycle’ of IIH. By restoring a physiological pressure gradient between the venous system and the subarachnoid space, venous sinus stentingA minimally invasive surgery during which a metallic mesh in the shape of a tube (stent) is placed in the sinus. Click the term to read more (VSSA minimally invasive surgery during which a metallic mesh in the shape of a tube (stent) is placed in the sinus. Click the term to read more) allows us to break this cycle and in many cases effectively relieve the symptoms related to elevated intracranial pressure (ICPincreased intracranial pressure Click the term to read more). However, IIH symptoms may recur in about 10 % of patients8. In most of these relapsed cases, a stenosis of the stent-adjacent ipsilateral transverse sinus and/or of the proximal third of the superior longitudinal sinus (SLS) is found, which seems to re-trigger the pathological loop of IIH9.
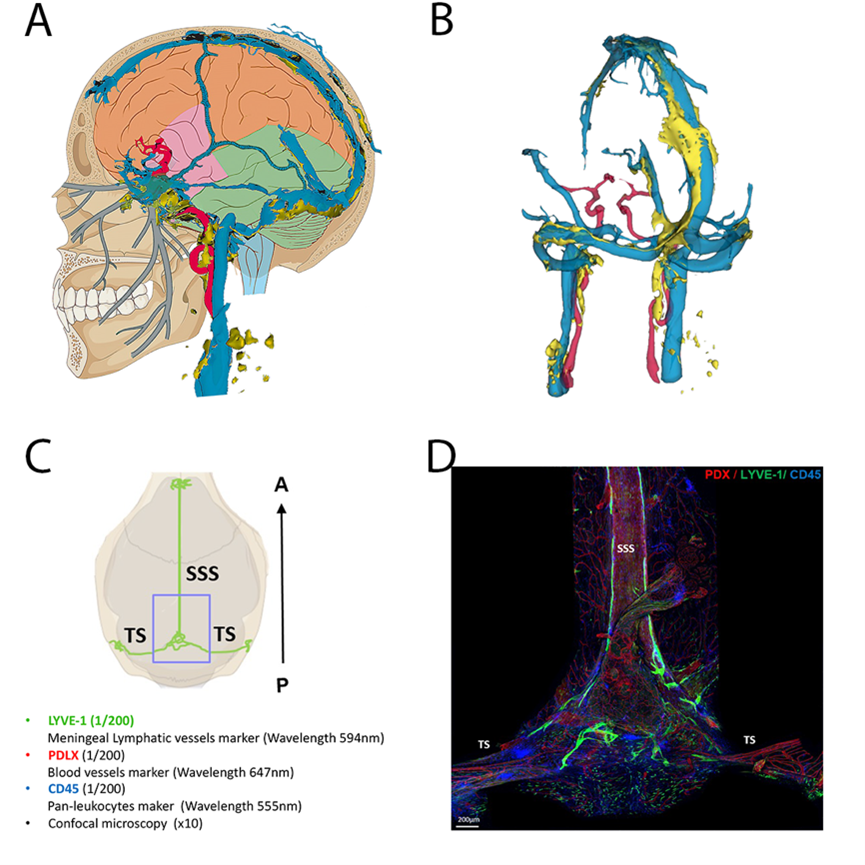
Techniques of exploration of the venous sinuses and parasinuses in humans and mice
A: schematic representation of the veno-lymphatic system in humans. Blue: dural venous sinuses, yellow: dural lymphatic system
B: 3D reconstruction of the veno-lymphatic system using MRI acquisition in humans according to the technique described in Jacob et al. Jexp Med 2022
C and D: Exploration of the parasinus in mice using immunofluorescence labeling on whole mounts preparations (courtesy to Marie-Renée El Kamouh, Paris Brain Institute)
BACKGROUND
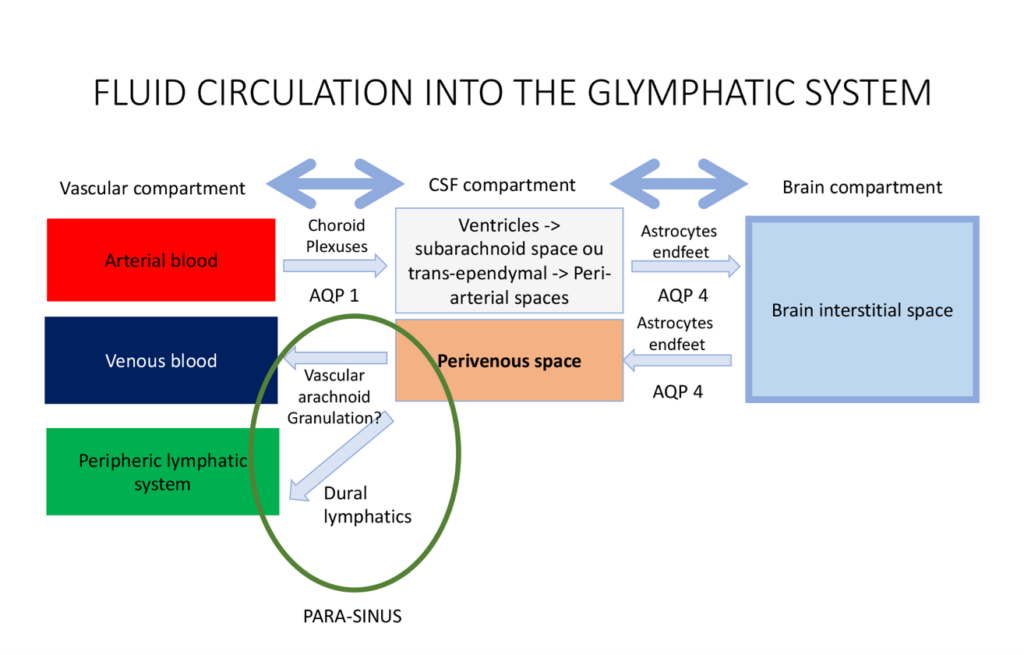
The Glymphatic System
In 2012, Iliff et al. identified the glymphatic system as “a brain-wide pathway for fluid transport, which includes the para-arterial influx of subarachnoid CSF into the brain interstitium, followed by the clearance of interstitial fluid along large-caliber draining veins”3. This process is preferentially activated during sleep10, and is driven by a combination of arterial pulsatility, respiration, and pressure gradients4, 11. The exchange of water molecules between the three compartments of the brain (i.e. the blood, the CSF and the brain parenchyma) is mediated by water-channel transporters called Aquaporins (AQP). CSF is continuously produced by the choroid plexuses through osmotic- and pressure-gradients that drive the movement of water and ions from the blood to the ventricular lumen10. From the ventricles, the CSF then exits through the foramina of Magendie and Monro to reach the subarachnoid space10. From the subarachnoid spaces, CSF then enters the periarterial spaces, travelling from the cortex toward the deep white matter along the courses of the pial and perforator arteries10. Along with other metabolites, CSF is then filtered and driven from the periarterial space to the brain parenchyma10. The transport of water from CSF to the brain is mediated by another water-transporter, AQP-43. This continuous movement of CSF from the periarterial space into the brain parenchyma then drives convective bulk parenchymal fluid flow toward the perivenous spaces surrounding the large cortical veins3. Following this, the method of resorption of CSF from the perivenous spaces is still unclear. Two CSF outflow pathways have been described in humans: the venous outflow pathway and the lymphatic outflow pathway.
CSF outflows
While the venous CSF outflow pathway has historically been considered as the only way of resorption of CSF, the recent discovery of the dural lymphatics in humans has been another major paradigm shift12. To better describe the CSF outflow pathways, we first need to clearly distinguish two physiological roles of CSF: a mechanical role which plays a role in the regulation of ICP, and a metabolic one which plays a role in clearance of brain metabolites.
The venous CSF outflow pathway
It was historically believed that the venous resorption of the CSF occurs across the arachnoid villi and granulations. These anatomical structures are traditionally described as focal areas of protrusion of the subarachnoid space across the dura matter into the lumen of the dural sinuses. These “avascular granulations” also play a mechanical role of regulation of the ICP, as the flow of CSF across the granulation is dependent on the pressure gradient between the subarachnoid space and the venous blood of the dural sinus. In the light of the recent scientific findings concerning the glymphatic system, it seems that another type of granulations – the so-called “vascular granulation” – has probably been wrongly neglected over time. Previous pathological13, 14 and radiological studies14, 15, support that some arachnoid granulations may enter the dura mater to reach the lumen of the venous sinuses in close association with a major cortical vein. These “vascular granulations” could represent an anatomical and physiological connection between the perivenous space (draining the ISF from the glymphatic system) and the venous blood of the dural sinus14. Vascular granulations may therefore be involved in the excretion of the brain metabolites as one final exit pathway of the glymphatic system. The intrinsic molecular mechanisms of this filtration are however still unknown.
The lymphatic CSF outflow pathway
It was long believed that the CNS did not have a lymphatic drainage system. Ironically, an Italian anatomist called Paola Mascagni described meningeal-related lymphatic vessels in a landmark anatomical text in 1787, but her findings were discounted by the scientific community for more than 200 years16. In 2015, the presence of functional lymphatic vessels lining the dural sinuses was eventually demonstrated in murine brains2, 5. Two years later, Absinta et al went on to image these dural/meningeal lymphatics in both primates and humans1. They also seem to be involved in the clearance of the CSF (or ISF) from the glymphatic system2, 17, and also in the regulation of the ICP (through a direct reabsorption of the CSF from the subarachnoid space)5. Other work by Hoon Ahn et al, showed that CSF drains preferentially through a basal outflow pathway, with CSF tracers draining via skull base meningeal lymphatics to the deep cervical lymph node system. Anatomically, the lymphatic system of the brain could therefore be described as a drainage network extending from the dural sinuses to both eyes, tracking above the olfactory bulb, following the dural arteries and veins into the dura matter2, 5. The dural lymphatics finally join the skull base, discharging the CSF into the sheaths of the cranial nerves. The CSF is eventually excreted into the deep cervical lymph nodes and the systemic lymphatic circulation18.